Compressed hydrogen
Hydrogen can be stored in the gaseous state as compressed hydrogen (GH2) at pressures typically in the range 150 – 700 bar. Sizes of such tanks can be from a few liters up to hundreds of m3 containing tens of tons of H2. The transfer of gas from a large storage tank to the end-user’s utility tanks involves the transfer of hydrogen gas at high pressure into a nearly empty tank of low pressure.
Hydrogen gas has the peculiar property that it heats up as the gas expands from a high-pressure state into a volume where the gas has a lower pressure. Most other gases cools as they expend. Technically, this is called the Joule-Thomson effect. Such heating can lead to damaging of the walls of modern composite material storage tanks.
The heat generated during filling at ambient temperature of a smaller H2-tank must be removed during the filling process. This can be done by using heat exchangers in the filling line or by pre-cooling (e.g., to –40 oC) the high-pressure gas.
IFE has research activities related to transfer of compressed GH2 between storage tanks in safe, energy and time efficient ways. Thermodynamic modelling of the gas in the various states during the transfer is an important tool in this regard. However, dynamic processes during the GH2 flow may also be important during the depressurization on bunkering and may lead to large temperature differences inside a tank during filling process. Such dynamic processes require computational fluid dynamics simulation (CFD) models, which also is an important tool used in the hydrogen technology research at IFE.
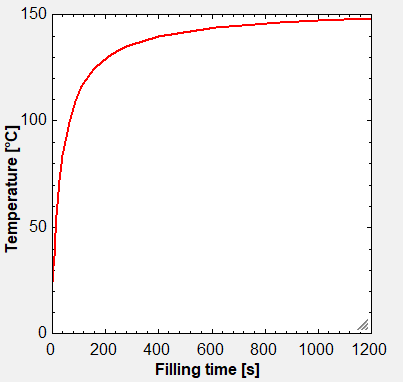
Fig. 2: Example showing temperature increase in end-user tank during filling at 20 oC of 620 kg of GH2 during 20 minutes from a 600 bar pressure storage tank. Final pressure in the small tank was 240 bar, and the gas was precooled to 5 oC during filling.
Projects:
FME HYDROGENi – Norwegian research and innovation centre for hydrogen and ammonia
INDY, Energy Independent and Efficient Deployable Military Camps
