FLI-AVAI
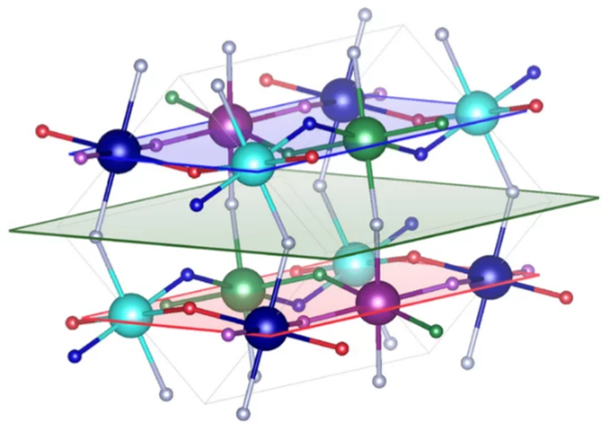
The FLI-AVAI project explores uncharted fluorides with unique d4 or d9 electron configurations, revealing their fascinating properties driven by structural Jahn-Teller deformations.
The optical, magnetic, and electronic properties of functional solids, such as oxides or fluorides, are governed by a delicate interplay between atomic arrangement (crystal structure), chemical bonding, and electronic properties for the involved transition metals. In the FLI-AVAI project, we study a special group of fluorides that are not previously investigated in great detail due to challenges with preparation (synthesis) and handling (air sensitivity). The studied fluorides have as common denominator that they contain transition metals with the special d4 or d9 electron configurations, which induce a structural Jahn-Teller deformation and thereof interesting physical properties. Similar electron configurations are found in important oxides, based on Mn(III) and Cu(II), which display properties such as magnetoresistance and superconductivity. The FLI-AVAI project is focused on fluoroperovskites of Cr(II) and Cu(II) with Jahn-Teller active electron configurations. We have developed a new synthesis method that allows access to these compounds in large quantities, which therefore enables the project. We will study how chemical modifications of the compounds, and how external stimuli, as temperature, magnetic field, and pressure, influence the crystal structure and properties.
Partners and external resources
Hydrogen Department IFE
Paul Scherrer Institute